What are Medical Device User Needs?
User needs are the foundation to developing an effective and safe medical device. While critical to the product development process, they are often misunderstood from the engineering and product management perspective. No fault to the engineering team as this lack of understanding generally comes from unclear definitions and terms being used interchangeably.
The FDA provides fairly brief guidance around Design Controls, more specifically paragraph (c) on Design Inputs outlines just a few points on the requirements of Needs and Inputs. But this is rather lacking, so in 1997 the FDA released an official guidance docuemnt, “Design Control Guidance for Medical Device Manufacturers”. That is where we get the famous waterfall diagram.
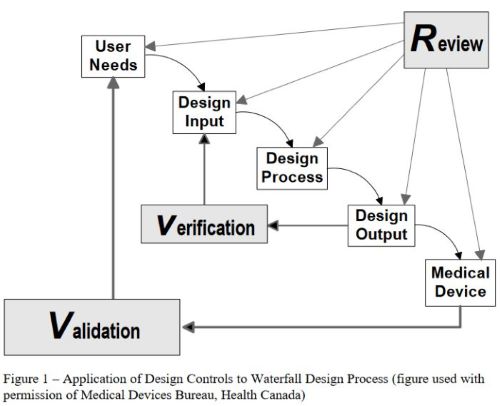
This is the first time the FDA starts to separate “User Needs” and “Design Inputs” as they are referred to in CFR 820.30. This guidance document does a pretty good job of outlining the FDA’s perspective on Design Controls, hence why it hasn’t been updated since 1997. In this guidance the FDA begins to draw the connection between User Needs and Intended Use(s) by stating; “design validation encompasses verification…to address whether devices produced in accordance with the design actually satisfy user needs and intended uses”. Just as a reminder this is what technology looked like in 1997.

It is fair to say that the interpretation of this guidance has evolved over time. Later in 2015 the FDA published a presentation on Design Controls called, “FDA Small Business Regulatory Education for Industry”. While this is not official guidance, its intent is to provide additional context on Design Controls within a Quality System. Slide 8 of this presentation outlines this on Design Inputs;
“Ensure requirements are appropriate by addressing user needs and intended uses in terms that are measurable.”
There is emphasis that Requirements and User Needs are two distinct entities, and that Requirements are responsible for addressing User Needs and Intended Uses of a device. The FDA already provides a very clear definition on what an Intended Use means in their eyes, so now we can begin to draw an understanding of how the three interact with one another. Let’s summarize some definitions.
- Intended Use: Defined as the general purpose of the device or its function, encompasses the indications for use.
- Requirements: Physical, chemical, digital, and performance characteristics or properties of a device that are used for the device design.
- Design Output: The product and its specifications.
We still don’t have a clear definition of what a “User Need” is. This definition is important as we consider traceability and decomposition in a larger context, and more importantly Validation for the design. A proposed definition;
- User Needs: A statement that describes a need, a problem, a challenge, or desire from the perspective of the user. Stated in qualitative language that can be objectively validated.
Why is this definition important?
- Well written User Needs provide clarity of what is Validated for the device.
- Having a clear strategy for Validation testing allows the team to reduce the risk of repeating costly testing of certain design elements.
- Defined User Needs provide clear traceability to Requirements (design inputs), risks, and testing artifacts.
Summary
User Needs are an essential piece to the design and development process, not only for compliance, but to ensure a safe and effective device. By providing a clear definition of what User Needs are, similar to Requirements, we are able to more clearly develop and document them. It becomes more clear to Engineers and Product Managers what is meant when the term User Needs is brought up in a design review. User Needs will serve as the basis for design Validation, tightly related to the Intended Use of the device, and having this definition will help us better assess the device.